The Role of CT in the COVID-19 Pandemic—Top 5 Takeaways
We’d like to start off by expressing our heartfelt gratitude to the men and women in healthcare who are braving this storm, putting themselves and their families at risk for their commitment to the health and well-being of their patients‒ we see you, we support you and we want to do our part to help you.
Even though we may be in isolation, we are not alone, and we will overcome this challenge together. #IsolatedButNotAlone #StopTheSpread
As an imaging-based lung AI company, we have been closely monitoring research, news, and expert consensus statements related to the role of CT in COVID-19 in this rapidly evolving situation. We have also been directly engaged with researchers and clinicians on the topic. In the spirit of sharing our learnings, here is our round-up of our most significant takeaways on this topic (at the time of publishing).
Top 5 Takeaways for the Use of CT in COVID-19
1. Early sensitivity results from February 2020― CT vs RT-PCR testing as a diagnostic tool
Early articles showed that CT imaging had higher sensitivity in detecting COVID-19 than RT-PCR testing (98% CT vs 71% RT-PCR). [2] With the RT-PCR test having a lower sensitivity between 60-70%, what this means is that if 1000 people infected with the virus are tested, 300 will come back as negative even though they are in fact positive. These people, thinking they are negative, can go on to infect other people. Thus, ultimately it has been recommended that all patients with negative RT-PCR tests who exhibit symptoms should assume they are positive and self-quarantine for 14 days.
In these early studies, this information, and the long waiting time to receive RT-PCR results, is why researchers initially concluded that CT should be used as the primary screening tool for COVID-19. [1]
However, the lack of CT specificity in diagnosing COVID-19 over other viral infections that cause acute lung injury as well as the logistical issues (see takeaway #2) in the use of CT for screening has ultimately led to CT screening becoming inadvisable.
Another limitation pointed out by other blog posts such as this one from Dr. Luke Oakden-Rayner addresses the potential of selection bias and lack of controls in these studies leading to CT’s higher sensitivity score.
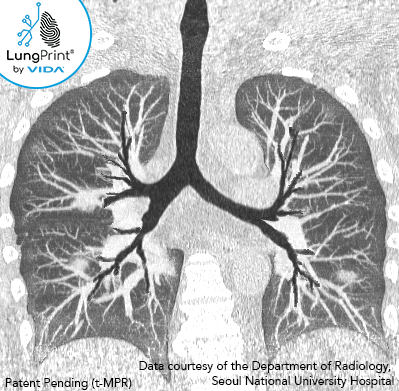
The image above is a VIDA topographic MPR slab view of a 50-60-year-old with confirmed COVID-19.
As of March 31, a new 2-5 minute serological test received an emergency FDA approval. The initial reporting indicates a sensitivity of 99% and a specificity of 91%. Given the novelty/recentness of the approval, no data is yet available on its performance in clinical use. [10]
2. March 2020― CT is not recommended for first-line screening for COVID-19
After these initial studies, and as the fluidity of the situation has progressed, neither the CDC nor the ACR (nor most radiology societies) has recommended the use of CT as a first-line test in screening for COVID-19. Why?

Depending on when the patient is imaged, results have shown that within the first 0-2 of infection up to 50% of confirmed patients may have normal features on CT imaging. [3-5]
In addition, cross-contamination between infected and non-infected patients utilizing the same CT scanner has been a concern as well as the potential exposure of staff and other patients in getting a possible COVID-19 patient to and from the scanner.
Cleaning and decontamination of the CT scanner can be time-consuming and place an undue strain in an already overworked system to provide repeated and thorough cleaning between possible COVID-19 patients and non-COVID-19 patients.
A recent paper in the Journal of the American College of Radiology provided an outline of four measures that radiology departments can take to control the spread of COVID-19 which includes establishing a "fever CT" protocol. With the four measures in place, over 3,000 people with fever underwent CT exams between January 21 and March 9 with no radiology staff showing signs of being infected. [9]
3. While screening may not be recommended, the RSNA acknowledges that CT does play a role
RSNA recently released an expert consensus article, endorsed by the ACR and STR, anticipating the use of CT for COVID-19. This article indicates the importance of radiologists in recognizing possible COVID-19 features for incidental findings and in the event that persons under investigation of COVID-19 require scanning for clinical management. [7]
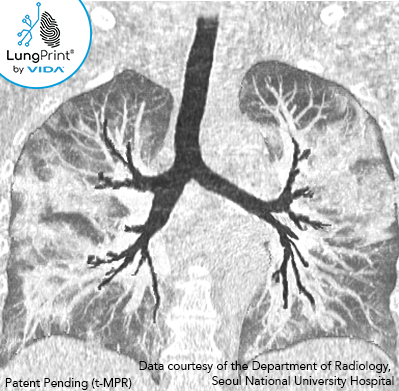
Typical imaging features of the COVID-19 infection include:
- Ground-glass opacities with or without consolidation. Nodular (or round) ground-glass opacities and “crazy paving” patterns have also been described.
- The distribution of the ground glass opacities in the early course of the infection has been characterized as bilateral (affecting both lungs), lower lobe, and peripheral focal or multifocal in approximately 50–75% of patients. [5]
- As the disease progresses into more moderate and advanced stages, crazy paving and consolidation are reported as the dominant CT findings. [5]
The image above is a VIDA topographic MPR slab view of a 60-70-year-old with confirmed COVID-19.
"At this time, CT screening for the detection of COVID-19 is not recommended by most radiological societies. However, we anticipate that the use of CT in clinical management as well as incidental findings potentially attributable to COVID-19 will evolve. We believe it important to provide radiologists and referring providers guidance and confidence in reporting these findings and a more consistent framework to improve clarity."
Radiological Society of North America Expert Panel [7]
4. Standardized reporting of possible COVID-19 features is recommended when discovered on imaging
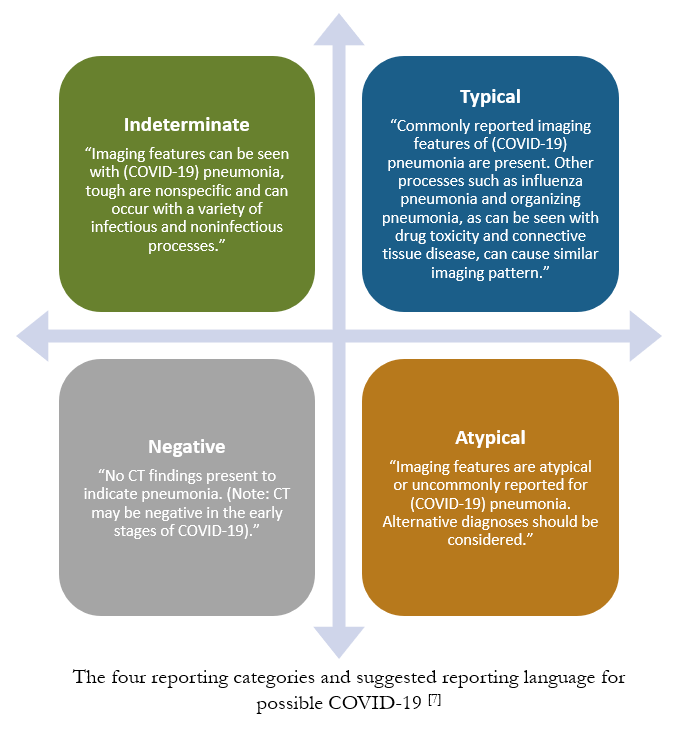
Due to CT’s lack of specificity and possible overlap of CT features with other viral causes of acute lung injury, the RSNA has provided recommendations for standardized reporting of possible COVID-19 imaging features.
The goal of structured reporting for possible COVID-19 is to aid radiologists in recognizing the findings, to decrease reporting variability, to increase confidence in reporting findings that may be attributable to COVID-19, and to initiate enhanced communication between clinical stakeholders. [7]
The recommended standardized reporting includes 4 categories: typical, indeterminate, atypical and negative. [7]
5. Longitudinal CT assessment and chronic lung disease
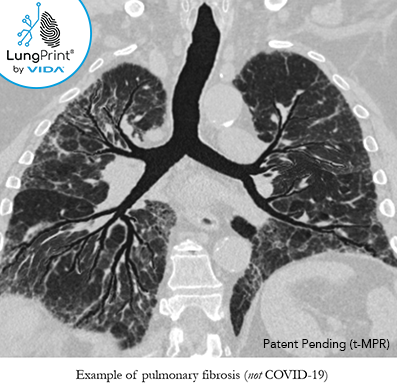
There have been reports of longitudinal assessment in recovered COVID-19 patients with signs of residual pulmonary fibrosis, as similarly seen in the early 2000's outbreak of severe acute respiratory syndrome. [8]
Even though CT has not been recommended for screening, as previously stated in #3, it has been reported that CT can be useful for assessing these patients for personalized management and monitoring response to treatment.
CT imaging features that have been reported include diffuse fibrosis with traction bronchiectasis. There may be potential for antifibrotics to treat these patients.
The image above is a VIDA topographic MPR slab view and is not a COVID-19 case, but an example of a patient with pulmonary fibrosis and traction bronchiectasis.
Our team at VIDA is eager to help healthcare workers and families across the world address this COVID-19 pandemic. We will continue to monitor research and industry societies for evolutions in the role of CT for COVID-19 patient management.
Contact us at fightcovid19@vidalung.ai for more information.
Other Resources
- UCSF resources to support mental health during this outbreak
- NEJM collections of articles
- NIH updates
- Netflix for those with "stay at home" orders
References
[1] Ai T, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 Feb 26:200642. doi: 10.1148/radiol.2020200642.
[2] Fang Y, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020 Feb 19:200432. doi: 10.1148/radiol.2020200432.
[3] Bernheim A, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020 Feb 20:200463. doi: 10.1148/radiol.2020200463.
[4] Pan F, et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020 Feb 13:200370. Doi: 10.1148/radiol.2020200370.
[5] Kanne J, et al. Essentials for Radiologists on COVID-19: An Update—Radiology Scientific Expert Panel. Radiology. 2020 Feb 27:200527. doi: 10.1148/radiol.2020200527.
[6] Wang Y, et al. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology. 2020 Mar 19:200843. doi: 10.1148/radiol.2020200843.
[7] Simpson S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology. 2020 Mar 25: 200152. doi: 10.1148/ryct.2020200152.
[8] Zhang P, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Research. 2020 Feb 14: 8(8). doi: 10.1038/s41413-020-0084-5.
[9] Huang Z, et al. The battle against coronavirus disease 2019 (COVID-19): emergency management and infection control in a radiology department. Journal of the American College of Radiology; 0(0). doi: 10.1016/j.jacr.2020.03.011.
[10] FDA authorizes new two-minute serological test kit to detect novel coronavirus. Business Wire. 31 March 2020. source